Cleanroom News, Cleanrooms
When is a Pharmacy Not a Pharmacy? Or ‘A Horse of a Different Color.’
Back in the ‘Old Country,’ polo is considered the ‘sport of kings.’ On any given day, weak sunshine bathing the pitch in a pale golden glow, the upper crust of the English horsey elite will gather for this centuries-old game of speed and tactic. Dukes and earls rub shoulders with up-and-coming titans of industry and their winsome trophy wives as the battle for dominance of the puck is played out on a meticulously manicured lawn. And when the match concludes and the horses are stabled, players and crowd adjourn for a glass of Pimms or some sweet tea, and a round of cucumber sandwiches.
At least that is the delicately romanticized image of polo.
But back in 2009 in Ocala, Florida, at the U.S. Open championship match the spectators’ appetite for post-game nibbles – whether cucumber or otherwise – was severely compromised. In front of the group of horrified onlookers, twenty-one of the competing horses suddenly and apparently inexplicably dropped dead. The horses – the Lechuza Caracas polo team – were owned by Victor Vargas, a Venezuelan entrepreneur, and three of the players, and they all wanted answers.
In the equestrian world, a horse’s worst enemy just might be a condition called rhabdomyolysis. Associated with a high-carbohydrate diet and lack of the mineral selenium, rhabdomyolysis can result in life threatening skeletal muscle degeneration with horses affected if they engage in strenuous physical exertion after a period of comparative inactivity. In the case of the polo ponies, for instance, after spending time in pre-game training following the forced idleness of transport from Venezuela to Florida.
If rhabdomyolysis is a potential issue for equine athletes, veterinarians outside of the US will commonly prescribe Biodyl, a compound produced by animal health product company Merial, headquartered in Duluth, Georgia. But although Merial manufactures Biodyl for international markets, it is not approved by the Federal Food and Drug Administration (FDA) for use within the US. So, seeking to replicate the supplement – an injectable solution containing ‘metabolic constituents (adenosine triphosphoric acid or ATP, magnesium and potassium aspartate, sodium selenite and vitamin B12) for debility, convalescence and myopathies’ – the veterinarian in charge of the polo ponies, Dr. James Belden, engaged the services of a compounding pharmacy, Franck’s Labs, then one of the largest compounding labs in the country.(1) And in doing so, he unwittingly set in motion the chain of fatal events that would culminate in the loss of the 21 horses.
Following the incident, an early article on ScienceBlogs.com initially speculated that the amount of the mineral selenium – which should have been measured in micrograms – may have been miscalculated as milligrams.(2) It would be a relatively simple mistake to make – a tired pharmacist misreading smeared or sloppy handwriting could easily have mistaken ‘mcg’ as ‘mg.’ And, although an essential element, selenium is highly toxic at extremely low dosages, with patients experiencing respiratory distress, renal failure, tachycardia, tremors, and ultimately heart attack.(3) And, according to veterinary reports and eye witness account, this certainly seems to be what happened to the ponies. An immediate investigation found the prescribing veterinarian to be not guilty in connection with the deaths, leaving the blame squarely upon the shoulders of pharmacists within Franck’s Lab. And unfortunately for the organization, it was not to be the last time the lab would be in trouble.
“…Investigators had uncovered serious flaws in their injectable drug production.”
Even as the investigation into the equine deaths progressed, Franck’s Lab continued to draw fire from critics within the industry and from the FDA for other reasons. In a warning letter dated July 9, 2012, the FDA cautioned the pharmacy that their investigators had uncovered serious flaws in their injectable drug production. Franck’s Labs produced Brilliant Blue G (BBG), a staining solution that is introduced into the vitreous cavity of the eye in order to stain – and thereby highlight – the internal limiting membrane (ILM) of the retina. In patients with retinal detachment or cellular proliferation of this membrane, macular holes may develop causing vision loss. When injected into the eye with an irrigating fluid, BBG allows an ophthalmic surgeon to clearly see the otherwise transparent membrane and remove it, a process known as ILM peeling.(4) And given that this solution is injected into a patient’s eyeball, it is safe to assume that the product should be absolutely sterile and free of contamination, per the standards set for aseptic processing.
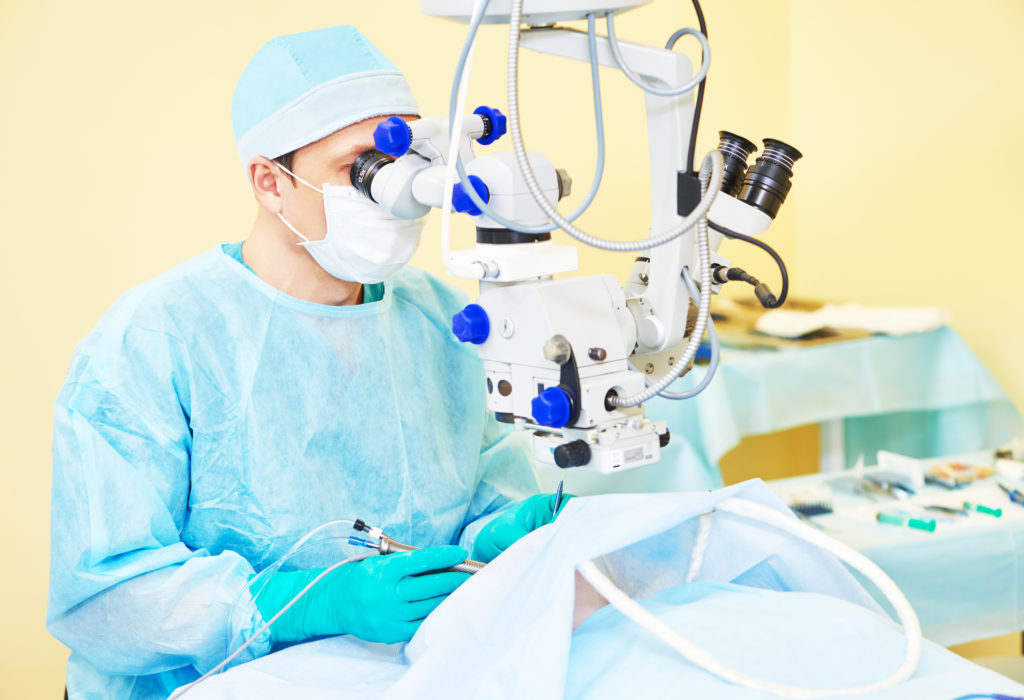
Unfortunately for Franck’s Labs – and any patients who received their BBG products – this was not the case.
“…observed flunking Basic Aseptic Compounding 101…”
In the 2012 warning letter, the FDA reports the adulteration of the product with ‘filthy, putrid, or decomposed substance’ and that solutions were ‘prepared, packed, or held under insanitary conditions whereby they may have been contaminated by filth, or whereby they may have been rendered injurious to health.’(5) These adulteration charges were significant. An FDA lab test revealed the presence of a contaminant that was matched to patients who developed fungal endophthalmitis after having been given the BBG injection. These patients, already suffering compromised vision, were subjected to the increased risk of further loss by the very treatment designed to improve their condition.(6) And furthermore, the fungal contaminants, along with bacteria, were found in multiple locations, including within one of the ISO Class 5 laminar flow hoods and inside of the ISO Class 7 cleanroom. Pharmacy employees were observed leaving and re-entering sterile areas without changing their personal protective equipment (PPE) as well as touching the outside of their gloves with bare hands. In essence, Franck’s Lab’s employees were observed flunking Basic Aseptic Compounding 101 in the immediate line of sight of the FDA…
Franck’s Labs ultimately issued a recall for affected products but by then it was too late. More than 30 patients were impacted and the decision was made to close down operations. In July of 2012, the business was purchased by Wells Pharmacy Network and all of Franck’s Labs and retail establishments quietly slipped out of the spotlight.(7)
Well, that would have been the case but for the matter of insurance. Let’s go back to the polo ponies again…
Following the deaths of the animals, the owners filed an initial claim for their financial loss with insurance agency Diamond State Insurance Co. A second one followed against Franck’s Labs, this one with the aim of establishing their liability given that the company was in the business of ‘designing, formulating, compounding, manufacturing, selling, and distributing veterinary medications and nutritional supplements.’(8) So far, this seems to be a fair analysis. However, Franck’s Labs’ insurers refused to defend their client, insisting they (the insurance company) had ‘no duty to defend or indemnify the insureds per a professional services exclusions in the policies.’(9) In effect, the labs’ insurer argued that it was not responsible for bodily injury or property damage arising out of the ‘rendering or failure to render professional health care services as a pharmacist.’(10) Which brought into question whether a compounding pharmacy was indeed a ‘pharmacy’ if its products were destined for the non-human market. And this is where the situation started to get seriously sticky.
In hearing the case of Cincinnati Ins. Co. v. Quorum Mgmt. Corp., 2016 WL2937461, Florida’s federal court decided to get back to basics. Seeking a legal definition of the term ‘pharmacist,’ it established that this must include a person or an entity that compounds preparations for a one-time medical use, not only for those engaged in mass production. Furthermore, the district court also sought to determine the meaning of ‘professional health care services,’ deciding that the definition pivoted not on the end user or patient but upon the nature of the services rendered… and with those definitions legal history was made for patients – both human and non-human.
In March of this year, jurors in West Palm Beach, Florida, awarded more than $2.5million to the owners and to the insurer of the polo ponies who died as a result of gross human error. Criminal negligence was established in each step of the process, with Franck’s Labs’ technician Judy Faison making the initial transcription error, staff pharmacist Anthony Campbell failing to correct the mistake, and verification pharmacist Nefertiti Abdullah ignoring problems in creating the compounded product. In the proceedings, it was shown that the procedural errors of improper compounding identified by the FDA were mirrored at other corporate levels, in a company trusted to create custom medical substances. And this is why Franck’s Labs, formerly the largest compounding pharmacy in the nation, needed to be out of business – to protect future patients – both human and non-human – from sloppy, unprofessional, and potentially fatal practices.
“…sloppy, unprofessional, and potentially fatal practices….”
If you’d like to read more, check out our earlier articles ‘Compounding the Problem’ and ‘When Honey is Not So Sweet.’ And if you have thoughts on the safety of compounding pharmacies, we’d love to hear them! Just submit them in the section below.
References:
- http://scienceblogs.com/terrasig/2009/04/24/biodyl-francks-pharmacy-and-fl/
- Ibid
- http://www.news-medical.net/health/Selenium-Toxicity.aspx
- http://retinatoday.com/2011/04/global-perspectives-brilliant-blue-g-for-ilm-staining-and-peeling/
- http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm312645.htm
- http://emedicine.medscape.com/article/1204298-overview#a2
- http://www.thehealthlawfirm.com/blog/posts/franck-s-pharmacy-closes-its-doors-compounding-lab-becomes-wells-pharmacy-network.html
- http://www.lexology.com/library/detail.aspx?g=5506e3d3-3068-41a9-9e00-7e525947d39d&utm_source=Lexology+Daily+Newsfeed&utm_medium=HTML+email+-+Body+-+General+section&utm_campaign=Lexology+subscriber+daily+feed&utm_content=Lexology+Daily+Newsfeed+2016-08-05&utm_term=
- Ibid
- Ibid
Additional references:
http://scienceblogs.com/grrlscientist/2009/04/23/pharmacy-admits-their-mistake/
http://www.foxnews.com/story/2009/04/23/florida-pharmacy-reportedly-admits-medication-mistake-in-death-21-polo-ponies.html
https://en.wikipedia.org/wiki/Biodyl


















Pingback: Compounding Pharmacies: Exploring the Insanitary/Unsanitary Conundrum - Cleanroom News | Berkshire Corporation